Keratin Materials
Cosmetics, Hair care & growth,
Leather industry, Animal feed,
Tissue engineering, Controlled released & delivery,
Vascular graft Wound healing, Hemostasis, Bone regeneration, Peripheral nerve
repair,...
Keratin ([1][2])
is one of a family of fibrous structural proteins. It is the key
structural material making up hair, horns, claws, hooves, and the outer layer of human skin. Keratin is also the protein that protects epithelial cells
from damage or stress. Keratin is extremely insoluble in water and organic
solvents. Keratin monomers assemble
into bundles to form intermediate filaments, which are tough
and form strong unmineralized epidermal
appendages found in reptiles, birds, amphibians, and mammals.[3][4] The
only other biological matter
known to approximate the toughness of
keratinized tissue is chitin.[5][6][7].
https://en.wikipedia.org/wiki/Keratin
Examples of occurrence
Keratin filaments are abundant in keratinocytes in the cornified layer of the epidermis; these are proteins which have undergone
keratinization. In addition, keratin filaments are present in epithelial cells
in general. For example, mouse thymic epithelial cells (TECs) are known to react
with antibodies for keratin 5, keratin 8, and keratin 14. These antibodies are
used as fluorescent markers to distinguish subsets of TECs in genetic studies of
the thymus.
- the α-keratins are found in all
vertebrates. They form the hair (including wool), stratum corneum, horns, nails, claws and hooves of mammals and the hagfish slime
threads.[4]
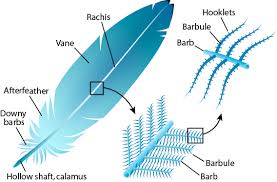
- the harder β-keratins are found only in the sauropsids, that is all living reptiles and birds.
They are found in the nails, scales, and claws of reptiles, some reptile shells (Testudines, such as tortoise, turtle, terrapin), and in the feathers, beaks, and claws of birds.[11] (These
keratins are formed primarily in beta sheets. However, beta sheets are also found in
α-keratins.)[12]
Additionally, the baleen plates of filter-feeding whales are made of keratin.
|

Horns such
as those of the impalaare
made up of keratin covering a core of live bone. |
|
Keratins (also described as cytokeratins) are polymers of type I and type II intermediate filaments, which have only
been found in the genomes of chordates (vertebrates, Amphioxus, urochordates).
Nematodes and many other non-chordate animals seem to only have type VI intermediate filaments, lamins, which have a long rod domain (vs. a short rod
domain for the keratins). |
|
|
|
Keratins are naturally derived proteins that can be fabricated into several
biomaterials morphologies including films, sponges and
hydrogels. As a
physical matrix, keratin biomaterials have several advantages of both natural
and synthetic materials that are useful in tissue engineering and controlled
released applications. Like other naturally derived protein biomaterials, such
as collagen, keratin possess amino acid sequences, similar to the ones found on
extracellular
matrix (ECM), that may interact with
integrins showing their
ability to support cellular attachment, proliferation and migration. The ability
of developing biomaterials that mimic ECM has the potential to control several
biological processes and this is the case for keratin which has been used in a
variety of biomedical applications due to its biocompatibility and
biodegradability.
https://pdfs.semanticscholar.org/ab50/ecefd0eb689b0cbd09ddb0589aa865ad1c64.pdf
Keratin Application Market Analysis
Extraction of keratin from keratinaceous substrates
Keratins are removed from the cortex first by using chemicals to break the
disulfide bonds that are prevalent in keratinized tissues. The alpha and
gamma-keratins are converted to their non-crosslinked forms by oxidation [22,
2426] or reduction [22, 27-29], during which cystine is converted to either
cysteic acid or cysteine, respectively. The free proteins extracted with
denaturating solvents produce a solution that can be purified by filtration and
dialysis.
Keratin-based biomaterials fabrication
The interest of using keratin as a biomaterial in medical applications is based on several key properties that
contribute to the overall physical, chemical and biological behavior of these
biomaterials. Extracted keratin proteins have an intrinsic ability to
self-assemble and polymerize into fibrous and porous films gels and scaffolds.
The spontaneous selfassembly of keratin has been studied extensively at both
microscale [35-37] and macroscale levels [38]. Furthermore, the presence of cell
adhesion sequences, arginine-glycine aspartic acid (RGD) and leucine-aspartic
acid-valine (LDV) on the keratin protein derived from wool and hair, makes
keratin biomaterials able to support cell attachment and growth.
Keratin Films and Coatings
Keratin films can be prepared by solvent casting. This technology is becoming
increasingly attractive for the production of films with extremely high quality
requirements. The advantage of this technology includes uniform thickness
distribution, maximum optical purity, and extremely low haze and is a technique
easy to use. The ability of keratin solution to self-assemble into films was
described by Yamauchi et al. [56] and, the physicochemical properties and
biodegradability of the solvent-cast keratin films were evaluated. Pure keratin
films presented low mechanical strength but the addition of glycerol resulted in
transparent films, with increased mechanical strength, flexible and
biodegradable both in vitro (trypsin) and in vivo (subcutaneous implantation in
mice) [56]. Furthermore, these films proven to promote and increased cell
adhesion and growth when compared to collagen and glass.
Keratin 3D-Constructs
The ability of extracted keratin to self-assemble into three dimensional porous
structures has led to their development as scaffolds for biomedical
applications. The sponge scaffolds were fabricated by lyophilization of an
aqueous keratin solution after controlled freezing. This resulted in sponges
with homogeneous porous microstructures. Lyophilization or freeze-drying
technique is based upon the principle of sublimation. The protein solution, of
desired concentration, is frozen and solvent is removed by lyophilization under
the high vacuum. Porous structures are formed from the voids left by the removal
of the solvent. Thus, the frozen solvent acts as porogen to produce porous
materials. The pore size can be controlled by the freezing rate and pH; a fast
freezing rate produces smaller pores.
Keratin-based drug delivery systems
Drug delivery which takes into consideration the carrier, the route of
administration and the target, has evolved into a strategy of processes and
devices designed to enhance the efficacy of therapeutic agents through
controlled release. For many drug applications controlled drug delivery has even
become a prerequisite to achieve therapeutic efficacy and/or avoid adverse side
effects [87, 88]. Controlled drug delivery systems are not only to protect and
stabilize the incorporated drug but also help to maintain significant local
levels for sustained therapeutic response at low frequency of administration.
Biomaterials for controlled drug delivery systems have to meet several
requirements.
A variety of polymers have been investigated for drug delivery purposes However,
there remains a need for biomaterials that can be highly controlled in terms of
composition and sequence, structure and architecture, mechanical properties and
function. To address these requirements, the exploration of keratin as a
biomaterial for controlled drug delivery has widely expanded over the last few
years. The most common and easiest way of incorporating drugs into keratin
biomaterials is by dissolving or mixing them directly into the keratin solution
before processing. The challenge of this method is to ensure that there is no
detrimental impact of the fabrication process on the integrity and bioactivity
of the drug. Keratin can be used to increase the release in highly hydrophobic
and non-degradable systems. The release rate can be modulated by film
composition and that the mechanism is dominated by film degradation and
diffusion [92]. In this way, keratin can be used to increase the release in
highly hydrophobic and non-degradable systems. The incorporation of drugs into
nanoparticles is another option. It was shown that higher release rates are
obtained at intracellular level (higher GSH concentration) with efficient
internalization showing the promising applications of keratin-g-PEG as drug
carriers for cancer therapy.
Keratin in biomedical applications
Keratin have a strong potential for development as clinically relevant
biomaterials because they are abundant, bioactive and a realistic source of
autologous proteins.
Ocular surface reconstruction:
The results suggested that keratin films could represent the replacement of the
amniotic membrane in ophthalmology because keratin films are more transparent
and stiffer than AM with similar levels of corneal epithelial cells attachment
and proliferation.
Hemostatic agent:
Keratin hydrogels for the treatment of acute myocardial infarction, promoting
angiogenesis. It was hypothesized that keratin hydrogel has the ability to
adsorb fluid and bind cells to act as an effective hemostatic agent.
Nerve tissue regeneration:
The studies revealed that keratin biomaterial is neuroconductive and contain
regulatory molecules capable of enhancing nerve tissue regeneration by enhancing
the activity of Schwann cells.
Wound healing:
Cross-linked keratin powder, films and hydrogels showed significant proliferation of wound healing cell lines like
microvascular endothelial cells, keratinocytes and fibroblasts. Moreover,
incubation of keratin materials with lymphocytes (T cells) and activated
lymphocytes showed, respectively, no proliferation and normal growth, indicating
that keratin materials are nonimmunogenic and that the body’s normal
cell-mediated immune response is not inhibited by keratin materials.
These were also applied to wounds on animals (rats) and humans, and a faster
healing of the wounds treated with keratin materials was observed and, in the
human model, with reduced pain [47, 49]. It was investigated the biological
mechanism underlying the observed clinical benefits of keratin-based products as
wound treatments [109]. The results suggested that the beneficial effects of
keratin are related to its positive effects on re-epithelialization via
stimulation of keratinocyte migration and production of collagen type IV and
VII.
|
|
|
|